New Oral Anticoagulants
Properties of an ideal anticoagulant:
- Oral administration
- Rapid onset of action/rapid offset of action
- Wide therapeutic range
- Predictable therapeutic effect with fixed or weight-based dosing
- No food or drug-drug interactions
- No monitoring required (but the ability to monitor if desired)
- Well defined pharmacokinetics in presence of renal/hepatic disease
- Easily reversible
- Cost effective
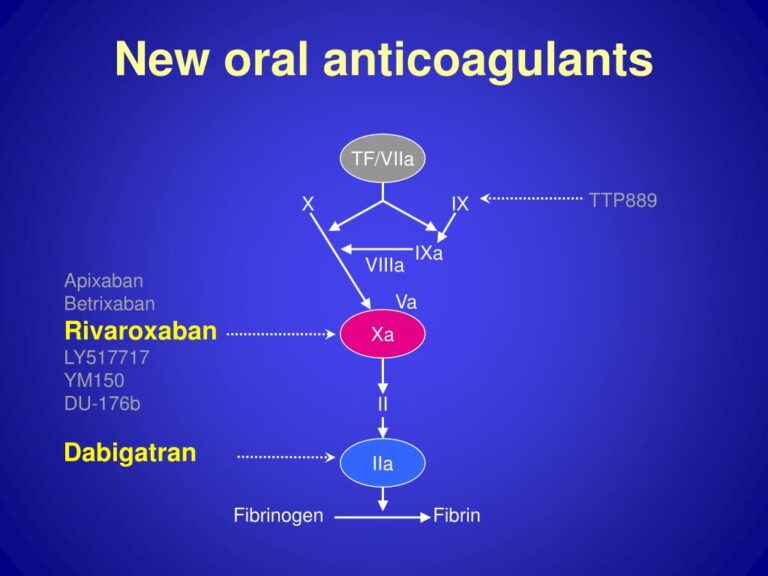
A. Direct thrombin (Factor IIa) inhibitors:
- Dabigatran(Pradaxa)
Oral direct thrombin Inhibitor. In October 19, 2010 it was approved by U.S. FDA for the prevention of stroke in patients with nonvalvular AF. In February 2011 it was added by American College of Cardiology Foundation and American Heart Association to their guidelines for management of nonvalvular AF (Class IB recommendation)
Pharmacokinetics: Ingested orally it is a competitive and reversible DTI. The maximum antiticoagulant activity starts 2-3 hours after ingestion. Its half life is 12–17 hours. Dabigatran is 35% protein bound. Metabolism is by conjugation and elimination is mostly renal (80%) and biliary(20%). It is contraindicated in patients with CrCl <30ml/min
Main side effect is dyspepsia (10%) and the disadvantage with dabigatran is that no antidote is available. Once a bottle of dabigatran is opened, the medication expires after four months because the drug is affected by humidity. Hence the bottle cap contains a desiccant to reduce the humidity.
Drug interactions:
- PPI’s reduce the absorption by 20-30%
- Drug excretion through P-glycoprotein pumps is slowed in patients taking P-gp pump inhibitors such as Quinidine, Verapamil, Clarithromycin, Amiodarone, thus raising plasma levels of dabigatran
- P-gp inducers such as Rifampicin, Carbamazepine or Phenytoin lead to decreased dabigatran concentrations
- Long term anti-platelet drugs (aspirin/clopidogrel) doubles the incidence of major bleeding events
- Long term NSAIDs can cause more bleeding episodes
- Antidepressants such as SSRIs and SNRIs also interact with dabigatran
Indications and dosage:
- VTE Prophylaxis during Hip/Knee replacement
- Dabigatran 110-mg capsule taken one to four hours after end of operation
- Continues with 220 mg (two 110-mg capsules) OD for 28-35 days after hip replacement/for 10 days after knee replacement
- Lower dose is advised in patients with
- Moderate kidney problems
- Patients >75 years age
- Patients also taking amiodarone, quinidine or verapamil
B. Factor Xa inhibitors
- Fondaparinux (Arixtra):
Fondaparinux is a synthetic pentasaccharide Factor Xa inhibitor. It mediates its effects indirectly through antithrombin III and is selective for factor Xa. Route of administration is subcutaneous.
Phrmacokinetics: Bioavailability of fondaparinux is 100% and the peak plasma time is 2-3 hrs. The half life is 17-21 hours. Fondaparinux is 94% protein bound (AT III). Metabolism of Fondapariux is by renal excretion in unchanged form. Hence, renal excretion precludes its use in patients with renal dysfunction.
The advantage of Fondaparinux over LMWH/UFH is that risk for Heparin-induced thrombocytopenia (HIT) is substantially lower.
Indications and dosage:
1.Treatment of DVT/PE:
- <50 kg: 5 mg SC /Day
- 50-100 kg: 7.5 mg SC /Day
- >100 kg: 10 mg SC /Day
2.Prophylaxis of DVT/PE:
- >50 kg: 2.5 mg SC /Day
3.Heparin-Induced Thrombocytopenia
- DVT prophylaxis (2.5 mg SC/Day) in patients with history of HIT
4.Acute Coronary syndrome
- 5 mg SC/Day
Location
- Maharashtra and Goa
- secretaryvsi@gmail.com
- +1 2123431725
Quick Menus
Newsletters
Be the first to know about exciting new visits,special event and much more
Copyright © 2023 By IMorse Technology Pvt. Ltd.

.png) +
+